Projects
Projects
Bromodomain Inhibitors
Cancer
Slowing tumour growth and disease progression by targeting bromodomain and extra-terminal (BET) domain-containing proteins.
Epigenetic alterations, such as histone acetylation, regulate the fate of cells, and are often behind the changes leading to tumour growth. The bromodomain and extra-terminal (BET) domain-containing proteins bind to acetylated histone residues and therefore play important roles in the cellular events leading to cancer.
There are four BET bromodomain family members: BRD-2, BRD-3, BRD-4, and BRD-T. Whilst pan inhibitors (i.e. inhibitors that target all / multiple family members) are in clinical development, there are significant potential risks associated with pan-BET inhibition. Specific inhibitors targeting individual members, and in particular BRD4, would therefore be of great value and could be used to treat cancers or inflammatory diseases.
We have been using commercially available small molecule compounds to further dissect and understand the mechanism of action used by BET domain proteins in laboratory models. In parallel, we have been using innovative medicinal chemistry approaches to design and synthesise novel and specific inhibitors of these proteins, which will provide the basis of new therapeutic treatments.
We aim to further develop specific BET inhibitors to treat cancers and inflammatory diseases without the risks associated with pan-BET inhibition.
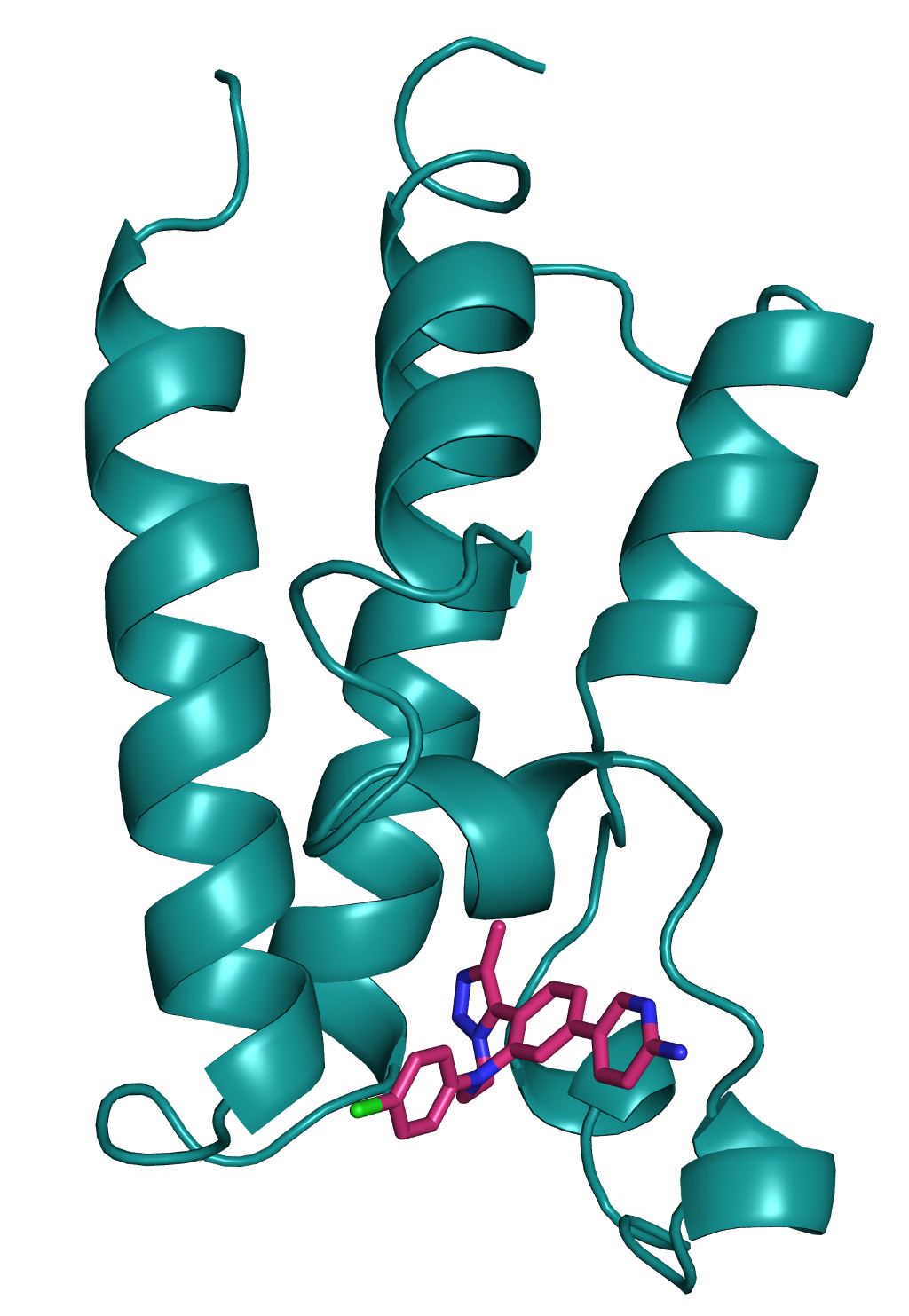
Pseudokinase platform
Cancer and Inflammatory Disease
Impeding cancer growth and inflammatory disease by targeting pseudokinases
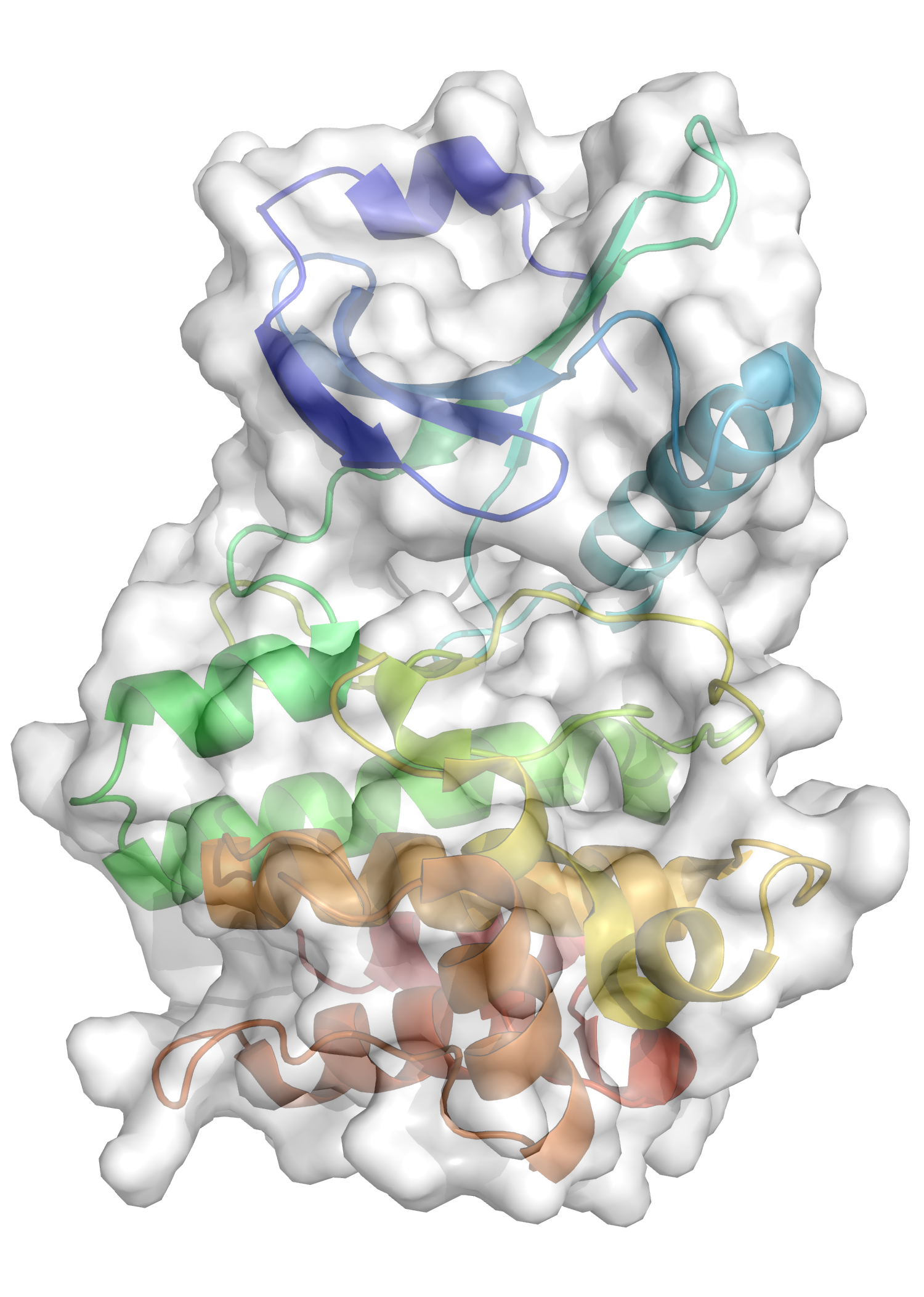
Pseudokinases are structurally similar to kinases but lack key elements that drive catalytic activity. There are over 50 pseudokinases and many have been implicated in the pathogenesis of cancers, autoimmune and inflammatory diseases.
They are purported to work in a number of ways including as: molecular switches; allosteric modulators; scaffold proteins and molecular sensors..
Catalyst Therapeutics has developed a compact and concise screening platform that delivers PoC compounds for the further elaboration of targets. This platform has already led to a license deal with a major pharma partner around a pseudokinase implicated in the pathogenesis of several inflammatory diseases.
We aim to further develop the platform with a view to identify, develop and commercialise novel pseudokinase small molecules modulators.

Artificial Intelligence Modelling of Small Molecule Targets
Decreasing cost and time to market using modern computational approaches.
Catalyst Therapeutics identifies molecules that bind and block specific targets using artificial intelligence, machine learning and quantum mechanics. We analyse the binding modes of these molecules and compare them with and exclude those that are similar to closely-related or associated molecules. This increases the likelihood of specificity.
Instead of each round of compound production and betterment occurring in a laboratory, incurring high costs and taking considerable time, we utilise an in silico, iterative, computational approach.
We design and utilise fragment-based mapping algorithms, such as the Multiple Fragment Molecular Dynamics (MFMD) approach, to map chemical functional groups associated with the target and identify ‘hotspots’ or differences that can be exploited to develop specific, selective inhibitors. These hits can be further refined using algorithms that integrate high-level quantum mechanics. The compounds are then synthesised and tested for binding, specificity, ADME properties and biological activity.
Traditional small molecule drug discovery involves several rounds of chemical structure synthesis / modification and biochemical, cellular or animal testing in order to refine a compound to insure optimal efficacy and selectivity. This approach will lessen the need for multiple rounds of Structure–Activity Relationship (SAR) analysis and significantly decrease the cost and time to market.

